Effect of olanzapine exposure on relapse and brain structure in patients with major depressive disorder with psychotic ... - Nature.com
Abstract
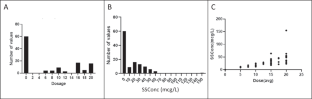
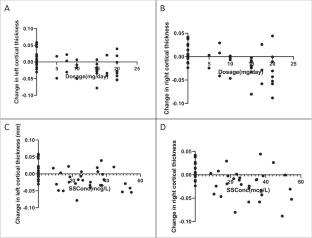
Some data suggest that antipsychotics may adversely affect brain structure. We examined the relationship among olanzapine exposure, relapse, and changes in brain structure in patients with major depressive disorder with psychotic features. We analyzed data from the Study of the Pharmacotherapy of Psychotic Depression II trial (STOP-PD II), a randomized, placebo-controlled trial in patients with psychotic depression who attained remission on sertraline and olanzapine and were randomized to continue sertraline plus olanzapine or placebo for 36 weeks. Olanzapine steady state concentration (SSC) were calculated based on sparsely-sampled levels. Rates of relapse and changes in brain structure were assessed as outcomes. There were significant associations between dosage and relapse rates (N = 118; HR = 0.94, 95% CI [0.897, 0.977], p = 0.002) or changes in left cortical thickness (N = 44; B = −2.0 × 10−3, 95% CI [−3.1 × 10−3, −9.6 × 10−4], p < 0.001) and between SSC and changes in left cortical thickness (N = 44; B = −8.7 × 10−4, 95% CI [−1.4 × 10−3, −3.6 × 10−4], p = 0.001). Similar results were found for the right cortex. These associations were no longer significant when the analysis was restricted to participants treated with olanzapine. Our findings suggest that, within its therapeutic range, the effect of olanzapine on relapse or cortical thickness does not depend on its dosage or SSC. Further research is needed on the effect of olanzapine and other antipsychotics on mood symptoms and brain structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: A Longitudinal, Randomised, Triple-blind, Placebo-controlled MRI Study
Increased global integration in the brain after psilocybin therapy for depression
Brain structural correlates of recurrence following the first episode in patients with major depressive disorder
Data availability
Data available upon request.
References
Gournellis R, Tournikioti K, Touloumi G, Thomadakis C, Michalopoulou PG, Michopoulos I, et al. Psychotic (delusional) depression and completed suicide: a systematic review and meta-analysis. Ann Gen Psychiatry. 2018;17:39.
Farahani A, Correll CU. Are antipsychotics or antidepressants needed for psychotic depression? A systematic review and meta-analysis of trials comparing antidepressant or antipsychotic monotherapy with combination treatment. J Clin Psychiatry. 2012;73:486–96.
Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, Alexopoulos GS, Rudorfer MV, et al. Effect of Continuing Olanzapine vs Placebo on Relapse Among Patients With Psychotic Depression in Remission: The STOP-PD II Randomized Clinical Trial. JAMA. 2019;322:622–31.
Voineskos AN, Mulsant BH, Dickie EW, Neufeld NH, Rothschild AJ, Whyte EM, et al. Effects of Antipsychotic Medication on Brain Structure in Patients With Major Depressive Disorder and Psychotic Features: Neuroimaging Findings in the Context of a Randomized Placebo-Controlled Clinical Trial. JAMA Psychiatry. 2020;77:674–83.
Ismail M, Straubinger T, Uchida H, Graff-Guerrero A, Nakajima S, Suzuki T, et al. MAP Bayesian modelling combining striatal dopamine receptor occupancy and plasma concentrations to optimize antipsychotic dose regimens in individual patients. Br J Clin Pharmacol. 2022;88:3341–50.
Lesh TA, Tanase C, Geib BR, Niendam TA, Yoon JH, Minzenberg MJ, et al. A multimodal analysis of antipsychotic effects on brain structure and function in first-episode schizophrenia. JAMA Psychiatry. 2015;72:226–34.
Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, Mulsant BH, Rudorfer MV, et al. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
APA. Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Text Revision. Washington, DC: American Psychiatric Association; 2000.
Williams JB. A structured interview guide for the Hamilton Depression Rating Scale. Arch Gen Psychiatry. 1988;45:742–7.
Spitzer R, Endicott J. Schedule for affective disorders and schizophrenia. 3 ed. New York, NY: New York State Psychiatric Institute: Biometrics Research; 1979.
Berk M, Ng F, Dodd S, Callaly T, Campbell S, Bernardo M, et al. The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract. 2008;14:979–83.
Meyers BS, English J, Gabriele M, Peasley-Miklus C, Heo M, Flint AJ, et al. A delusion assessment scale for psychotic major depression: Reliability, validity, and utility. Biol Psychiatry. 2006;60:1336–42.
Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–93.
Davies SJ, Mulsant BH, Flint AJ, Meyers BS, Rothschild AJ, Whyte EM, et al. The Impact of Sertraline Co-Administration on the Pharmacokinetics of Olanzapine: A Population Pharmacokinetic Analysis of the STOP-PD. Clin Pharmacokinet. 2015;54:1161–8.
Bigos KL, Pollock BG, Coley KC, Miller DD, Marder SR, Aravagiri M, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48:157–65.
Moriguchi S, Bies RR, Remington G, Suzuki T, Mamo DC, Watanabe K, et al. Estimated dopamine D(2) receptor occupancy and remission in schizophrenia: analysis of the CATIE data. J Clin Psychopharmacol. 2013;33:682–5.
Sumiyoshi T, Watanabe K, Noto S, Sakamoto S, Moriguchi Y, Okamoto S. Prospective Epidemiological Research on Functioning Outcomes Related to Major Depressive Disorder in Japan (PERFORM-J): Protocol for a Prospective Cohort Study. JMIR Res Protoc. 2018;7:e161.
Ho BC, Andreasen NC, Nopoulos P, Arndt S, Magnotta V, Flaum M. Progressive structural brain abnormalities and their relationship to clinical outcome: a longitudinal magnetic resonance imaging study early in schizophrenia. Arch Gen Psychiatry. 2003;60:585–94.
Ho BC, Andreasen NC, Dawson JD, Wassink TH. Association between brain-derived neurotrophic factor Val66Met gene polymorphism and progressive brain volume changes in schizophrenia. Am J Psychiatry. 2007;164:1890–9.
Vernon AC, Natesan S, Modo M, Kapur S. Effect of chronic antipsychotic treatment on brain structure: a serial magnetic resonance imaging study with ex vivo and postmortem confirmation. Biol Psychiatry. 2011;69:936–44.
Konopaske GT, Dorph-Petersen KA, Pierri JN, Wu Q, Sampson AR, Lewis DA. Effect of chronic exposure to antipsychotic medication on cell numbers in the parietal cortex of macaque monkeys. Neuropsychopharmacology. 2007;32:1216–23.
Konopaske GT, Dorph-Petersen KA, Sweet RA, Pierri JN, Zhang W, Sampson AR, et al. Effect of chronic antipsychotic exposure on astrocyte and oligodendrocyte numbers in macaque monkeys. Biol Psychiatry. 2008;63:759–65.
Bigos KL, Bies RR, Pollock BG, Lowy JJ, Zhang F, Weinberger DR. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16:620–5.
Fleisher AS, Truran D, Mai JT, Langbaum JB, Aisen PS, Cummings JL, et al. Chronic divalproex sodium use and brain atrophy in Alzheimer disease. Neurology. 2011;77:1263–71.
Acknowledgements
We thank the members of the STOP-PD II Study Group for their contributions. Members of the STOP-PD II Study Group are listed in the Supplementary Information.
Author information
Authors and Affiliations
Contributions
All authors made substantial contributions to the design of the study, data acquisition, data analysis, interpretation, and/or preparation of the manuscript. All authors participated in drafting and revising of the manuscript and approved the final version to be published.
Corresponding author
Ethics declarations
Competing interests
HKK has no financial relationships with commercial interests. ANV receives funding from the National Institute of Mental Health (NIMH), Canadian Institutes of Health Research, Canada Foundation for Innovation, Centre for Addiction and Mental Health (CAMH) Foundation, and the University of Toronto. NHN receives grant support from th...
Comments
Post a Comment